Describe Using a Chemical Equation the Breakdown of Hydrogen Peroxide
The balanced equation of the decomposition reaction of hydrogen peroxide is that 2H2O2 decomposes into the products 2H2O O2 g. While this is a catabolic reaction the rate at which it occurs is slow.
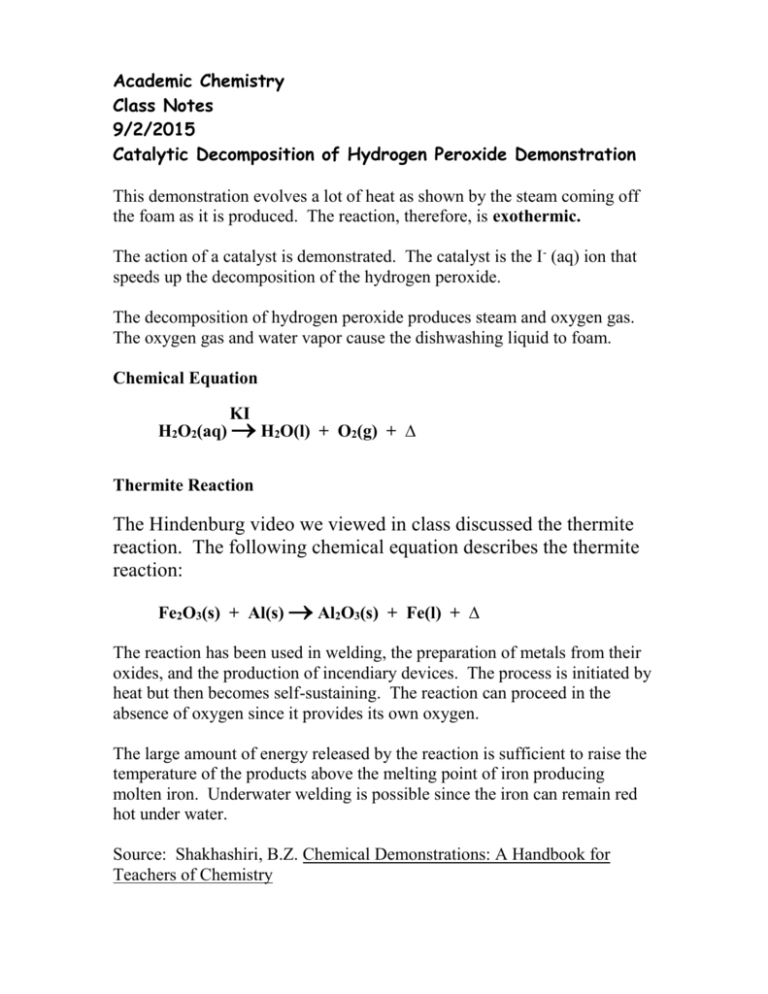
Decomposition Of Hydrogen Peroxide
Hydrogen peroxide undergoes disproportionation.

. Light and temperature affect the reaction rate. Hydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form it is a very pale blue liquid slightly more viscous than waterIt is used as an oxidizer bleaching agent and antiseptic usually as a dilute solution 36 by weight in water for consumer use and in higher concentrations for industrial useConcentrated hydrogen peroxide. The resulting products are water and oxygen gas.
2 H 2 O 2 aq --- 2 H 2 O l O 2 g enthalpy. A chemical hydrogen peroxide is shown to decompose to produce oxygen and water by capturing the gas evolved in. You could also write a word equation.
Hydrogen peroxide as a reducing agent in the presence of a strong oxidising agent in both alkali and acidic media and importantly oxygen is released every time. They promote the disproportionation of superoxide into oxygen and hydrogen peroxide which is then rapidly decomposed by the enzyme catalase to oxygen and water. As a result bottles of hydrogen peroxide you may purchase at the drug store are sold in.
You could describe this reaction by saying Hydrogen peroxide decomposes to form water and oxygen gas. How does hydrogen peroxide decompose. Reaction of the breakdown of hydrogen peroxide and its features Properties of hydroperite aka perhydrol.
The second step is the catalase breaking down another hydrogen peroxide molecule by releasing oxygen gas and water. Hydrogen peroxide has bound to the heme group and oxidized it to FeIV. Using hydrogen peroxide describe how symbols and subscripts are used to show a chemical formula.
The decomposition of hydrogen peroxide in the presence of iodide ion occurs in two steps. H 2 O 2 2H 2e- O 2 Acidic medium H 2 O 2 2OH 3H 2 O 2e- O 2 Basic medium 7. This is an experiment most students in chemistry lab are familiar with.
After this oxygen is given. 2 H2O aq catalase 2 H2O l O2 g hydrogen peroxide enzyme water oxygen gas. 2 2 H.
20 mL 30 hydrogen peroxide available from chemical supply establishments. Hydrogen peroxide is written as H202. Depending on the pH level.
H 2 O 2 aq I- aq H 2 O l OI- aq H 2 O 2 aq OI- aq H 2 O l O 2 g I- aq Materials Preparation. Although the cells are dead catalase still remains active. The activation energy of the reaction is about 75 kJmol in the absence of catalyst.
However to make the reaction perceptible at a. 40 30 Volume of hydrogen. The class of biological enzymes called superoxide dismutase SOD is developed in nearly all living cells as an important antioxidant agent.
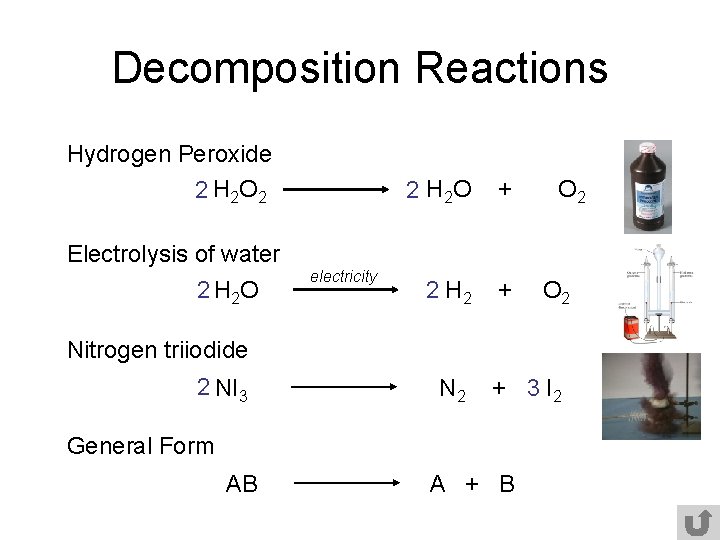
The equation for the reaction is Zns 2HCIaq ZnC12aq H2g An experiment is carried out using 012 g of powdered zinc an excess of 02 moldmž hydrochloric acid a temperature of 20 oc The volume of hydrogen collected in the experiment is measured at regular time intervals. A decomposition reaction occurs when one reactant breaks down into two or more products. 2 Educator answers Science.
Under higher temperatures and concentrations it decomposes to form water and oxygen. This can be represented by the general equation. AB A B.
Platinum metal catalysts can lower the. Why is it possible to use dead cells to study the function of this enzyme. Decomposition of hydrogen peroxide.
Breaks down hydrogen peroxide. Terms in this set 11 What reaction does catalase catalyze. A chemical formula shows the ratio of elements in a compound.
Now for the second part of the reaction. 2H2O2 -- O2g 2H2Ol How many molecules of water are produced from the decomposition of 34g of hydrogen peroxide H2O2. Hydrogen peroxide is a very reactive compound that can be used for a variety of reactions including bleaching and disinfecting minor wounds.
BaO 2 8H 2 Os H 2 SO 4 aq BaSO 4 s H 2 O 2 aq 8H 2 Ol. Catalase breaks down and destroys hydrogen peroxide in two steps. In pure form it is unstable and easily breaks down with the process often being accompanied by.
Share Tweet Send Deposit Photos Hydrogen peroxide is an unstable substance. What is the balanced chemical equation for the breakdown of. WHEN catalase is added to hydrogen peroxide there is an initial rapid evolution of oxygen which lasts for about two minutes depending on the peroxide concentration.
The following reaction will clarify this. Curve B shows the results obtained. Enzymes work by lowering the energy of activation.
Breakdown of hydrogen peroxide. Hydrogen Peroxide with a chemical equation of H2O2 looks very similar to the equation for water H2O however having an extra Oxygen atom compared to water but this changes the properties of Hydrogen Peroxide. The first step involves the catalase removing and binding one oxygen atom and releasing the rest of the hydrogen peroxide molecule as water.
Decomposition of hydrogen peroxide can be catalysed by other compounds such as transition metals like silver and platinum. Explanation including important chemical equations. However the decomposition takes place very slowly.
Both oxidation and reduction occur at the same time. For example hydrogen peroxide decomposes to form water H2O and oxygen gas O2. The enzyme has to go back to the Fe III form and reduce the second molecule of hydrogen peroxide to water.
Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen and the breakdown of water to hydrogen and oxygen. H 2 O 2 O FeIV-Enzyme -- H 2 O FeIII- Enzyme 2. Hydrogen peroxide water oxygen When you light a burner on your stove methane gas bursts into flames and.
When barium peroxide is acidified and the excess water is removed by the process of evaporation under reduced pressure we obtain hydrogen peroxide. Show the fully labeled balanced chemical equation for the decomposition of hydrogen peroxide.
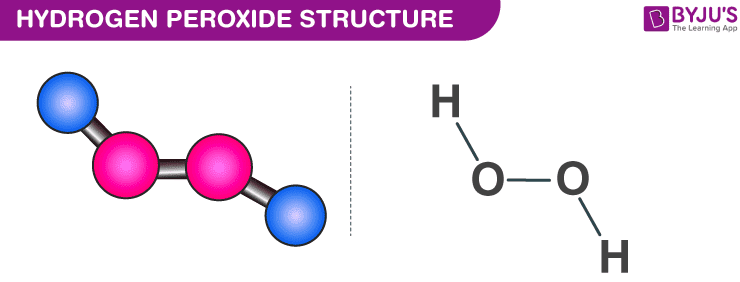
Hydrogen Peroxide H2o2 Structure Preparation Properties Uses
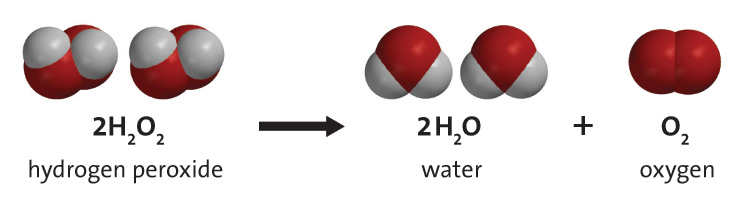
How Does A Catalyst Make Hydrogen Peroxide S Decomposition Quicker What Is Actually Happening Socratic

How To Write The Formula For Hydrogen Peroxide Youtube
Chemical Equations Reactions Chemistry A Chemical Reactions You

A Catalyst And The Rate Of Reaction Chapter 6 Chemical Change Middle School Chemistry

Question Video Using Word Equations To Describe The Decomposition Of Hydrogen Peroxide H2o2 Nagwa

Chemical Equations Chemistry Mrs Coyle Chemical Equations Represent Chemical Reactions Word Equations Skeleton Chemical Equations Balanced Chemical Ppt Download
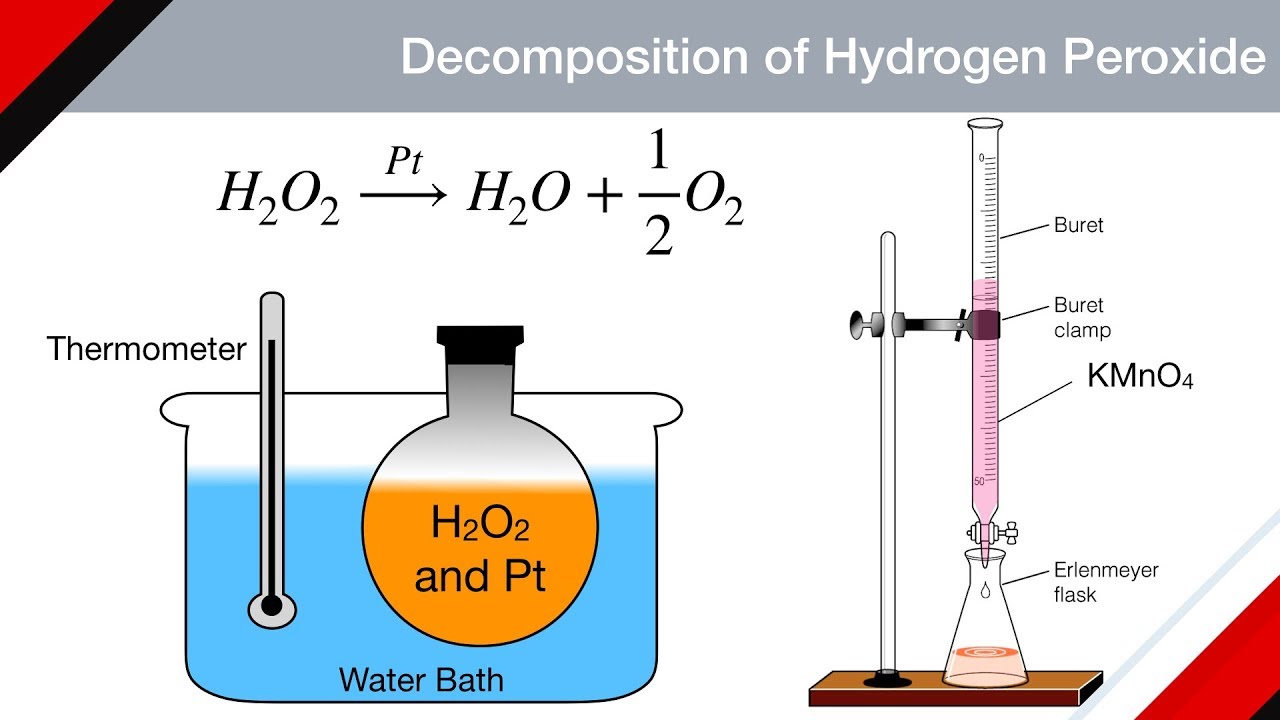
Kinetics Of Decomposition Of Hydrogen Peroxide Chemical Kinetics Physical Chem Youtube

Hydrogen Peroxide Structure Properties Uses With Questions Videos
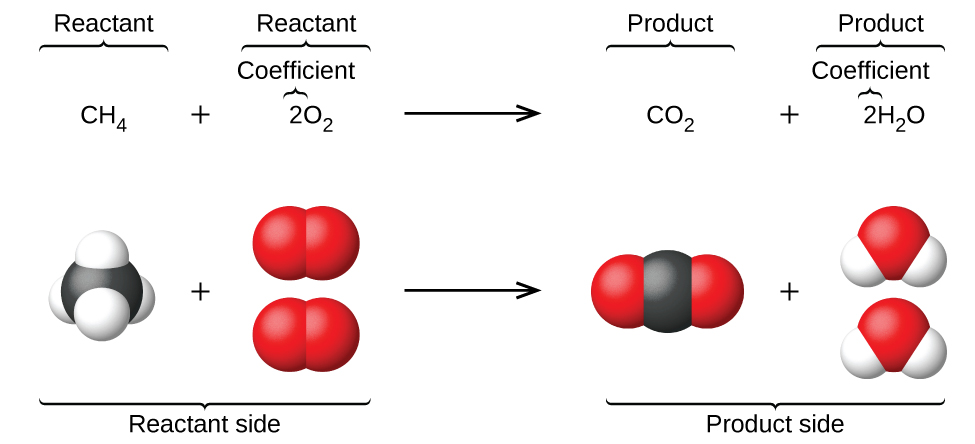
Writing And Balancing Chemical Equations Chemistry 2e

The Catalytic Decomposition Of Hydrogen Peroxide Ppt Download

Q16 In The Laboratory Preparat Lido
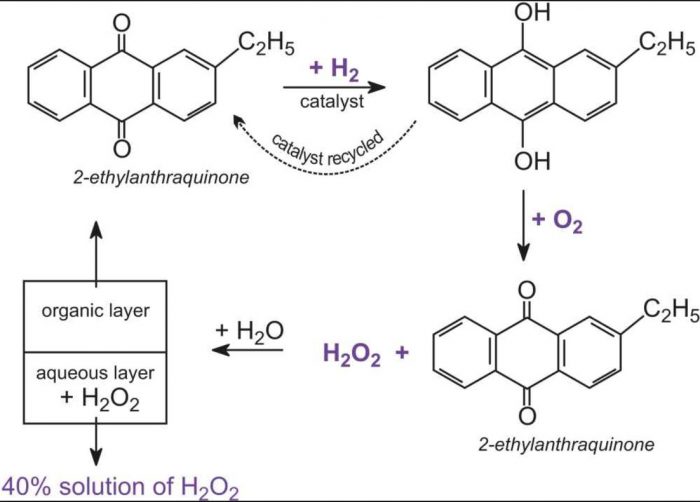
Hydrogen Peroxide Chemistry Class 11 Hydrogen
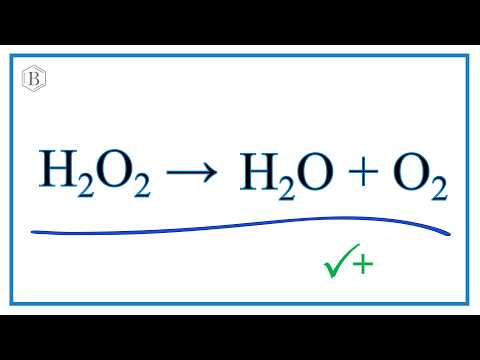
How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube

How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube
What Type Of Reaction Is Catalase And Hydrogen Peroxide Quora

Hydrogen Peroxide Preparation Properties Structure And Uses Definition Examples Diagrams

Pdf Decomposition Of Hydrogen Peroxide Kinetics And Review Of Chosen Catalysts
Comments
Post a Comment